Body Mass Index and Waist Circumference was Risk Factors for Increased Fasting Blood Sugar, and Hemoglobin A1c in Korean Adults without Diabetes Mellitus
Article information
Abstract
Purpose: This study confirmed the effects of body mass index (BMI) and waist circumference (WC) on fasting blood glucose (FBS) and hemoglobin A1c (HbA1c) in adults without diabetes mellitus (DM). Methods: The subjects were 4,659 adults (≥20 years), and data were extracted from the 6th National Health and Nutrition Survey 2015 (KNHANES VI-3). The subject’s data were analyzed using a complex sample t-test, χ2-test, and multiple logistic regression analysis (SPSS 24.0). Results: The incidence of the high FBS group was increased 1.60 times in males with a WC ≥90 cm and 1.78 times in females with a WC ≥85 cm. The incidence of the high HbA1c group was increased 1.54 times in those who were overweight and 2.22 times in those who were obese. The incidence of the high HbA1c group was increased 1.99 times in males with a WC ≥90 cm and 1.87 times in females with a WC ≥85 cm. Conclusion: This study presented evidence that interventions for BMI and WC should be included in DM prevention programs. It also suggested that these findings can be utilized for early detection of DM.
INTRODUCTION
Obesity is increasing worldwide and is recognized as being closely associated with chronic diseases, such as diabetes mellitus (DM) [1]. Obesity results in health problems caused by excessive fat accumulation [2]. The prevalence of adult obesity in Korea is 42.3% for male and 26.4% for women, central obesity is also increasing due to abdominal fat accumulation [3]. Obese adults have more than double the incidence of DM and rates of fasting glucose disorders are higher in obese adults [4]. There are more than 382 million people with DM worldwide. In approximately 15 years, it is estimated that approximately 10% of the world’s population will have DM [5]. Currently, the prevalence of adults with diabetes in Korea is 12.4% [6].
Body mass index (BMI) is used to measure obesity. Even if the BMI is normal, waist circumference (WC) or the waist-to-hip circumference ratio increases the risk of DM and metabolic syndrome. There is growing interest in the distribution of body fat, especially abdominal obesity [7]. Consequently, central obesity is a predictive factor for DM [8]. BMI increases, individuals are more vulnerable to DM [1]. Abdominal obesity may also be a predictor for DM [8] and WC has been used as an index of abdominal obesity [4].
In a study developed for Western populations, obesity was defined as a BMI >30 kg/m2 and being overweight was defined as a BMI >25 kg/m2 [2]. However, the World Health Organization’s classification criteria are not suitable for Asian populations [7]. It is more appropriate to define obesity for the Asian population as a BMI ≥25 kg/m2 [9]. Therefore, this standard was used in this study.
BMI is positively correlated with fasting glucose and hemoglobin A1c (HbA1c) as fat tissue increases the prevalence of DM [10]. The American Diabetes Association has suggested the measurement of fasting blood glucose (FBS) and HbA1c levels for the diagnosis of DM [11]. FBS reflects the level of glucose at the time of testing. HbA1c reflects the average blood glucose level for 3 months depending on the life of the red blood cells [12].
In previous studies related to obesity, accumulation of abdominal fat was considered to explain the relationship between obesity and metabolic syndrome [1]. WC is an independent and significant prognostic indicator for obesity-related DM [8]. In addition, in a comparative study on diabetes risk according to obesity type, the BMI (≥25 kg/m2) and WC (males ≥90 cm, females ≥85 cm) groups, the WC (males ≥90 cm, females ≥85 cm) groups were found to be factors influencing increased prevalence of DM, and the BMI (≥25 kg/m2) group was not a factor influencing the prevalence of DM [13]. Abdominal obesity and DM prevalence have been found to be related in both men and women [14]. Obesity according to BMI classification [10], Central obesity according to WC classification [8] as well as the type of obesity (General obesity and abdominal obesity group, general obesity group, abdominal obesity group) [13] are risk factors for DM. For DM prevention and early detection, it is necessary to determine the effect of general obesity and abdominal obesity on the increase of FBS and HbA1c, which are DM diagnostic indicators, in subjects without DM.
The purpose of this study was to identify the factors affecting FBS and HbA1c for early detection and prevention in patients prior to a DM diagnosis. Thus, in this study, subjects diagnosed with DM were excluded to rule out the outcomes of DM. This study aimed to determine the influence of BMI and WC on FBS and HbA1c in individuals without DM. This study also provides a basis for the early detection of DM.
METHODS
1. Design
This descriptive survey study investigated the effects of BMI and WC on FBS and HbA1c levels in adults without diabetes mellitus.
2. Setting and Samples
The subjects of this study were adults (>20 years), and their data were extracted from the 6th National Health and Nutrition Survey 2015 (KNHANES VI-3) data. The KNHANES VI-3 used a two-stage cluster sampling method to select representative survey subjects in Korea. The first-level extraction used a survey and the second-level extraction used the family. The survey consisted of a health interview, nutritional survey, and health examination. All participants underwent physical examinations by trained staff and they completed a questionnaire. This study analyzed 4,659 subjects aged 20 years and older who were not diagnosed with DM.
3. Variable measurement
1) FBS and HbA1c
Blood was collected by a trained professional after fasting for at least 8 hours. FBS was measured using the hexokinase UV (Hitachi Automatic Analyzer 7600-210, Hitachi, Tokyo, Japan). According to the FBS level, normal was defined as a FBS <100 mg/dL, an increased diabetes risk was defined as an FBS of 100-125 mg/dL, and a diabetes diagnosis was confirmed with an FBS of ≥126 mg/dL [11]. In this study, according to the classification criteria [11], FBS normal group (<100 mg/dL) and a high FBS group (100-125 mg/dL) were classified.
HbA1c levels were measured by high-performance liquid chromatography (Tosoh G8, Tosoh, Tokyo, Japan). According to the HbA1c levels, a normal level was <5.7%, an increased diabetes risk category was 5.7-6.4%, and a diagnosis of diabetes was ≥6.5% [11]. In this study, HbA1c normal group (<5.7%) and high HbA1c group (5.7-6.4%) were classified according to the criteria [11].
2) BMI
The BMI was the subject’s weight (kg) divided by height squared (m2). Height, and weight were measured in the morning after fasting overnight. In this study, normal weight (BMI <23 kg/m2), overweight (BMI; 23-24.9 kg/m2) and obesity (BMI ≥25 kg/m2) were used as classification criteria [9]. In a study developed for Western populations, obesity was defined as a BMI >30 kg/m2 and being overweight was defined as a BMI >25 kg/m2 [2]. However, the World Health Organization’s classification criteria are not suitable for Asian populations [7]. It is more appropriate to define obesity for the Asian population as a BMI ≥25 kg/m2 [9]. Therefore, this standard was used in this study.
3) WC
WC was measured in the middle between the last rib and iliac ridge. This was not the maximum WC [15]. The same measurement method was applied in this study. According to the criteria presented by the Obesity Society [16] and in this study, a WC ≥90 cm for males and WC ≥85 cm for females were used as abdominal obesity criteria.
4. Data analysis
The data in this study applied a complex sampling design analysis to reflect weights that were stratified and clustered. In the raw data of the National Health Nutrition Survey, there were missing values. Therefore, complete observations were only used data analysis. General characteristics indicated unweighted frequencies and weighted estimate rates. FBS and HbA1c differences were analyzed according to general characteristics. BMI and WC were analyzed using a complex sample t-test and Rao-Scott chi-square test. Complex sample multiple logistic regression analysis was performed to identify the variables that influence the increase in FBS and HbA1c levels. The adjusted odds ratios (aORs) and 95% CIs were calculated as dependent variables for FBS and increases in HbA1c (SPSS 24.0).
5. Ethical consideration
This study used KNHANES VI-3 data, which surveyed a large number of subjects nationwide. KNHANES VI-3 was previously approved by the Korea Centers for Disease Control and Prevention Institutional Review Board (IRB) (approval number 117002), and personal information was coded with a serial number to ensure the anonymity and confidentiality of the subject. This researcher has obtained permission to use KNHANES VI-3 data from the responsible institution.
RESULTS
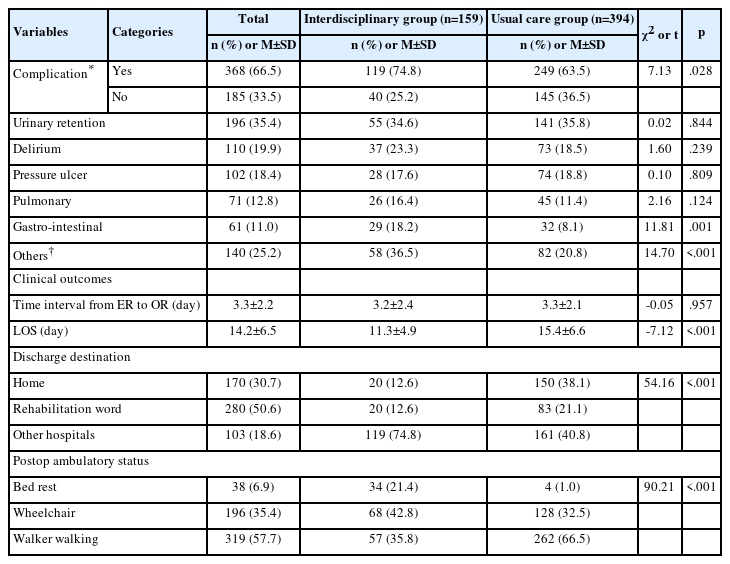
1. General characteristics
The participants’ average age was 45.77 years. Males represented 48.8% and females represented 51.2% of the participants. Regarding the participants’ educational level, beyond-college level represented the highest group at 39.7%. Regarding the economic level, the middle-high income group was the highest at 26.08%. Regarding the BMI classification, the participants’ average BMI was 23.81 kg/m2, 43.6% had a normal weight, 23.1% were overweight, and 33.4% were obese. The participants’ WC was 82.20 cm for males, 70.4% were less than 90 cm, and 29.6% were more than 90 cm. In females, 74.4% were less than 85 cm, and 25.6% were >85 cm. The average FBS was 96.13 mg/dL. The FBS normal group represented 74.7% of the participants and the high FBS group represented 25.3% of the participants. The average HbA1c was 5.49%. The HbA1c normal group represented 80.8% of the participants and the high HbA1c group represented 19.2% of the participants (Table 1).
2. FBS and HbA1c differences according to the general characteristics
The age of the participants was higher in the high FBS group (52.20 yr) than in the FBS normal group (43.38 years) (t=114.29, p<.001). Regarding age classification, 20-34 year-olds (34.1%) were the most common participants in the FBS normal group and 51-64 year-olds (34.4%) were the most common participants in the high FBS group (χ2=264.86, p<.001). In terms of sex, females (54.7%) were more common in the FBS normal group, and males (61.6%) were more common in the high FBS group (χ2=88.98, p<.001). For the educational level, the beyond-college level (43.4%) was the most common educational level in the FBS normal group and high school (36.6%) was the most common level in the high FBS group (χ2=113.15, p<.001).
The age of the participants was higher in the high HbA1c group (56.50 years) than in the HbA1c normal group (42.99 years) (t=124.45, p<.001). Regarding age classification, participants 35-50 years (35.7%) were the most common in the HbA1c normal group and participants 51-64 years (41.1%) were the most common in the high HbA1c group (χ2=511.64, p<.001). For the educational level, beyond-college level participants (43.9%) were the most common in the HbA1c normal group and high school level participants (34.5%) were the most common in the high HbA1c group (χ2=230.76, p<.001) (Table 2).
3. FBS and HbA1c differences according to the to the BMI and WC
The participants’ BMI was higher in the high FBS group (25.35 kg/m2) than in the FBS normal group (23.28 kg/m2) (t=202.30, p<.001). Regarding the BMI classification, a normal weight (50.0%) was most common in the FBS normal group and obesity (50.1%) was most common in the high FBS group (χ2=258.68, p<.001). The WC of the participants was higher in the high FBS group (87.55 cm) than in the FBS normal group (80.34 cm) (t=384.94, p<.001). Specifically, a WC ≥90 cm, the criterion for abdominal obesity in men, was found to be higher in the high FBS group (42.2%) than in the FBS normal group (24.4%) (χ2=62.05, p<.001). A WC ≥85 cm, the criterion for abdominal obesity in women, was found to be higher in the high FBS group (48.9%) than in the FBS normal group (19.3%) (χ2=182.98, p<.001).
The participants’ BMI was higher in the high HbA1c group (25.39 kg/m2) than in the HbA1c normal group (23.42 kg/m2) (t=168.30, p<.001). Regarding BMI, a normal weight (47.9%) was most common in the HbA1c normal group and obesity (51.8%) was most common in the high HbA1c group (χ2=190.88, p<.001). The WC of the participants was higher in the high HbA1c group (87.61 cm) than in the HbA1c normal group (80.85 cm) (t=286.67, p<.001). Specifically, a WC ≥90 cm was found to be higher in the high HbA1c group (48.6%) than in the HbA1c normal group (25.3%) (χ2=77.24, p<.001). A WC ≥85 cm was found to be higher in the high HbA1c group (50.0%) than in the HbA1c normal group (19.0%) (χ2=195.82, p<.001) (Table 3).
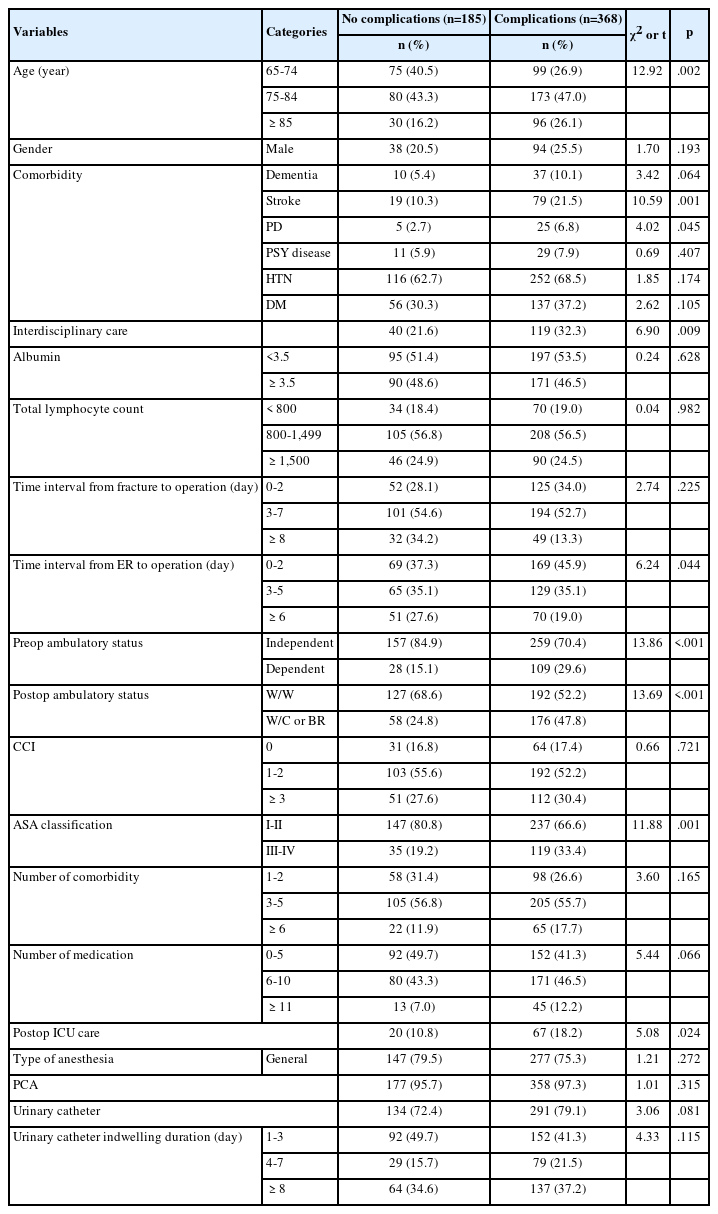
4. Predictive variables for increased the FBS and HbA1c
The factors influencing the high FBS group were WC, age, and sex. The incidence of the high FBS group increased 1.60 times (95% CI 1.15 to 2.24, p=.006) in males with a WC ≥90 cm and 1.78 times (95% CI 1.27 to 2.50; p=.001) in females with a WC ≥85 cm. On the other hand, it was found that being overweight or obese in the BMI classification did not influence the high FBS group. Regarding age, the incidence of the high FBS group increased 2.83 times (95% CI 2.12 to 3.785; p<.001) in the 35–50 years age group, 4.51 times (95% CI 3.33 to 6.15; p<.001) in the 51–64 years age group and 4.80 times (95% CI 3.53 to 6.54; p<.001) in the 65 years or older age group compared with the 20–34 years age group. The incidence of the high FBS group decreased by 0.69 times (95% CI 0.59 to 0.81; p<.001) more in females than in males.
The factors influencing the high HbA1c group were BMI, WC, age, and sex. Regarding the BMI classification, the incidence of the high HbA1c group was 1.54 times (95% CI 1.09 to 2.19; p<.001) higher in overweight participants, and 2.22 times (95% CI 1.46 to 3.36; p=.042) higher in the obese participants compared with the normal weight group. The incidence of the high HbA1c group increased 1.99 times (95% CI 1.54 to 3.18; p<.001) in males with a WC ≥90 cm and 1.87 times (95% CI 1.31 to 2.67; p=.001) in females with a WC ≥85 cm.
Regarding age, the incidence of the high HbA1c group increased 5.08 times (95% CI 2.44 to 10.56; p<.001) in the 35-50 years age group, 15.40 times (95% CI 7.43 to 31.93; p<.001) in the 51-64 years age group and 20.19 times (95% CI 9.56 to 42.66; p<.001) in the 65-years or older age group compared with the 20–34 years age group. The incidence of the high HbA1c group decreased by 0.75 times (95% CI 0.62 to 0.91; p=.005) more in women than in men (Table 4).
DISCUSSION
This study investigated the effects of BMI and WC on FBS and HbA1c in Korean adults undiagnosed with DM for early diagnosis and prevention. In this study, the factors affecting the FBS increase group were WC, age, and sex, and the factors affecting the HbA1c increase group were BMI, WC, age, and sex. Elevated FBS and HbA1c are used as indicators for a diagnosis of diabetes. HbA1c is a better indicator than FBS for the treatment of diabetic patients [10,12].
In the current study, BMI was a factor that influenced the high HbA1c group. However, it was not found to be an influencing factor in the increase of FBS. BMI is positively correlated with FBS [10] and HbA1c levels [1]. FBS increases with obesity [10], and obesity causes peripheral resistance to insulin-mediated glucose intake and decreases β-cell sensitivity to glucose. This is also an influencing factor for DM [21]. The probability of DM increases with an increasing BMI, and increases by 2.79 times in obese cases [17]. In the case of obesity among patients with DM, the HbA1c is not well controlled [22]. The mechanism by which obese individuals with an increased HbA1c level are associated with increased blood glucose is through weight gain [12]. Weight loss decreases the incidence of DM due to a decrease in blood glucose [23]. In this study, BMI affected HbA1c because excessive adipose tissue inhibits the regulation of blood sugar. Therefore, this researcher believes that it is essential to maintain a normal BMI to prevent DM.
In our study, BMI was not a factor in the high FBS group because Asians have more abdominal fat than Caucasians at the same BMI level [1]. It is thought that the effect on FBS is greater for WC than BMI. In DM patients, BMI affects an increase in FBS [10], because the subjects in this study were not diagnosed with DM, it is thought that different results were given.
In the present study, WC was a factor influencing high FBS and HbA1c levels. Specifically, FBS and HbA1c levels were increased in participants with abdominal obesity (male ≥90 cm; female ≥85 cm). WC and HbA1c were positively correlated, and WC was higher in participants with an HbA1c 5.7%-6.4% than those with an HbA1c <5.7% [24]. WC is associated with metabolism [25]. In a study of overweight (23.0-24.9 kg/m2) individuals, the probability of high-risk DM increased 1.014 times as the WC increased. In addition, even if obese (≥25.0 kg/m2) the probability of high-risk DM decreased 0.989 times as the WC decreased [24].
The DM diagnosis group had a higher WC than the non-DM diagnosis group. This means that Asians have more abdominal fat than that observed in a given BMI [26]. This abdominal visceral fat increases insulin resistance by the release of free fatty acids [27]. Therefore, WC was thought to be high in the DM group. HbA1c can be used as an index for predicting DM [28]. DM can be prevented by identifying it in high-risk DM groups in advance of a diagnosis. WC affects metabolic syndrome [26]. The risk of conversion to DM was increased with dyslipidemia and decreased insulin sensitivity in studies with normal and high-risk DM patients [29]. Therefore, it is necessary to measure, utilize, and manage WC to prevent DM.
In our study, age was a factor influencing high FBS and HbA1c levels. FBS and HbA1c increased with age and correlated with age. This indicated that age is an influencing factor for FBS and HbA1c [12] and the probability of DM risk increases with age [17]. Aging causes many changes in the human body. Muscle tissue decreases, thereby reducing glucose consumption and decreasing sensitivity to insulin receptors [18]. Thus, this supports the results of our study.
Sex was a factor influencing the FBS in the HbA1c increase group in the current study. This effect was reduced in females more than in males. HbA1c levels are lower in women [12]. A total of 29.7% of females and 32.4% of males were in the high-risk DM group. The proportion of females was low [17]. These results indicate that women control HbA1c better than men [19]. Women with a low BMI control blood glucose more successfully [20]. In addition, menstruation decreases hemoglobin levels in women, which is associated with rapid erythrocyte turnover [12], HbA1c is a form where blood sugar is attached to hemoglobin, and HbA1c levels are lower in women with decreased hemoglobin [17].
In this study, the influencing factor in the high FBS group was WC. BMI does not sufficiently reflect the difference in body fat mass between Asians and Westerners [7]. Asians have a higher risk of DM because they have a relatively higher visceral fat accumulation and insulin resistance than Westerners despite their low BMI [30]. Therefore, in our study, it was found that WC had a greater effect on DM risk when participants were overweight than when they were obese. The difference in the effects of BMI and WC on DM diagnostic indicators in adults without DM was confirmed in our study. Based on the results of the present study, BMI and WC interventions should be included in the early detection and prevention programs for diabetes.
CONCLUSION
In this study, BMI and WC were higher in the high FBS and HbA1c groups. BMI was a factor influencing HbA1c increase. WC was a factor influencing the high FBS and HbA1c groups.
In conclusion, DM prevention and early detection programs for Korean adults should include interventions for BMI and WC. This study is meaningful in confirming the difference between BMI and WC for FBS and HbA1c in Korean adults without DM. However, the limitation of this study is that it does not measure the effects of BMI and WC on insulin resistance, such as proinsulin. In future studies, it is essential to confirm the impact of BMI and WC on DM according to race, genetic factors, and insulin resistance. In addition, there is a need for research on the WC effect on the risk of DM in according to the level of obesity.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHORSHIP
HSL contributed to the conception and design of this study, performed the statistical interpretation, drafted the manuscript and critically revised the manuscript. All authors read and approved the final manuscript.