Effect of exercise interventions on sarcopenic obesity in middle-aged and older adults: a comprehensive review
Article information
Abstract
Purpose
This study examined the definitions, diagnostic criteria, and measurements of sarcopenic obesity and identified effective exercise interventions that improve cardiometabolic outcomes in middle-aged and older adults, in whom the prevalence of sarcopenic obesity is increasing.
Methods
This comprehensive review followed the principles of literature search, data extraction, and review, as described in the PRISMA 2009 guidelines.
Results
The 11 articles included in this study presented different concepts of sarcopenic obesity. Exercise interventions for sarcopenic obesity varied in their effects. Resistance exercise improved muscle mass and physical function, while aerobic exercise primarily impacted obesity and cardiometabolic indicators. Combined exercise had mixed results across indicators.
Conclusion
This study addressed a pressing public health concern in the context of an aging population, acknowledged the unique challenges of sarcopenic obesity, and attempted to clarify definitions and assessment methods, while identifying effective exercise interventions to reduce cardiometabolic risk. Sarcopenic obesity is a multifaceted condition with varying definitions and diagnostic criteria. Its association with cardiometabolic risk underscores the need for comprehensive assessments considering both muscle and obesity indicators. While exercise interventions hold promise for managing sarcopenic obesity, further research is required to establish effective strategies.
INTRODUCTION
Due to the improvement in nutrition and medical technology development, life expectancy has increased worldwide, and the proportion of the elderly population is also increasing. In South Korea, older adults over 65 comprise 15.7% of the total population in 2020. Moreover, the super-aged society will launch at 20.6% in 2025 [1]. The aging population leads to an increasing prevalence of chronic diseases and medical expenses [2].
These problems are related to the increased obesity rate. The obesity rate among the Korean population aged 60~69 and over 70 in 2019 was 37.3% and 34.3%, respectively [3]. In particular, obesity among older adults increases the risk of chronic diseases, such as hypertension, diabetes, dyslipidemia, and cardiovascular disease. These situations also increase medical expenses [4].
With age, body composition changes, such as muscle mass decreases and fat mass increases. The muscle mass decreases by 3%-6% every year after age 60 [5]. On the other hand, advanced age increases adipose tissue by 10% in men and 6% in women [6]. Muscle loss and increased fat characterize obesity in older adults. This condition is called Sarcopenic obesity [7]. Unlike obesity in general, which is characterized by excess body fat and often results from an imbalance between calorie intake and expenditure, sarcopenic obesity combines obesity with muscle loss, increasing muscle weakness and vulnerability to falls, posing a dual challenge [7].
However, The definition of sarcopenic obesity is not clear. The criteria for sarcopenic obesity vary according to the purpose of a study, and the measurement of sarcopenic obesity is also not standardized. Despite the variety of criteria and different measurement methods, sarcopenic obesity means satisfying both sarcopenia and obesity criteria simultaneously. Baumgartner was the first to define Sarcopenic obesity as the concept of sarcopenia and obesity [8].
Although Baumgartner introduced the concept of sarcopenic obesity in 1998 [9], it is still not recognized as actual obesity due to decreased muscle mass despite increased fat mass, so its risk is overlooked and not adequately managed. Those who are average or underweight tend to be less concerned with sarcopenic obesity. However, even a normal weight or underweight state can not be free from sarcopenic obesity. In terms of phenotype, sarcopenic obesity may not look like being obese. So, It is likely not to consider a need for management. There is a requirement to improve the awareness of sarcopenic obesity.
According to a systematic review of definitions and diagnostic criteria [10], muscle mass or strength, such as walking speed and handgrip strength, can define sarcopenia. Moreover, BMI, fat mass, visceral fat area, and waist circumference are obesity indexes. Various ways measure these parameters. Specifically, muscle mass can be presented by total muscle mass or appendicular lean mass in a ratio of weight, squared height, or BMI. Other indicators for muscle mass are fat-free mass index by upper arm or thigh muscle circumference. Thus, The definition, diagnostic criteria, and measuring methods for sarcopenic obesity are in chaos.
The prevalence varies from 6.1% - 22.5% for men and 7.3% - 21.1% for women, depending on the diagnostic criteria of sarcopenic obesity [11-14]. However, the prevalence of sarcopenic obesity increases with physical inactivity due to long-term sedentary lifestyles, nutritional imbalance related to insufficient protein intake, systemic inflammatory disease, and age [7,15,16]. In sarcopenic obesity, muscle loss and body fat increment create a negative synergistic effect, increasing the cardiometabolic risk such as diabetes, hypertension, and dyslipidemia [17]. Moreover, Sarcopenic obesity causes physical dysfunctions such as falls and gait disorders in daily life and consequently lowers the quality of life of older adults [18].
To manage obesity in general, limit calories and exercise to lose weight. However, with general obesity management for sarcopenic obesity, the loss of muscle mass may worsen. Therefore, it is necessary to apply an intervention to manage sarcopenic obesity differentiated from general obesity management. The need for obesity management through exercise and diet is highly recognized, and many studies have been conducted. However, the awareness and management of sarcopenic obesity are poor. Because of unstandardized definitions, diagnostic criteria, and measuring methods, the management intervention for sarcopenic obesity is unclear. Therefore, reaching a consensus on a definition, diagnostic criteria, and measurement is necessary to identify effective exercise methods for sarcopenic obesity discriminated from general obesity management.
This study aims to identify the effective exercise interventions improving cardiometabolic outcomes of sarcopenic obesity for middle-aged and older adults with an increasing prevalence through a comprehensive literature review. The study will examine the definitions, diagnostic criteria, and measurement of sarcopenic obesity and outcome variables to test interventions' effectiveness.
METHODS
This comprehensive review followed the principles of literature search, data extraction, and review of the PRISMA 2009 guidelines [19]. This comprehensive review conducted the study selection process rigorously and methodically. The selection criteria were established a priori to ensure the relevance of the chosen studies to the research question. This process included a comprehensive database search and a meticulous examination of titles, abstracts, and full texts to determine whether each study met the predefined inclusion and exclusion criteria. Furthermore, the methodology for study selection was informed by consultations with another experienced researcher to enhance the robustness of the selection process.
Eligibility criteria
Inclusion criteria were as follows: adults over middle-aged living in the community, sarcopenic obesity subjects, prescribed exercise interventions and physical activities related to the study (possibly dietary combined exercise interventions), and indicators that can confirm the effectiveness of interventions. Exclusion criteria were as follows: without full-text, subjects without sarcopenic obesity, unclear definition of sarcopenic obesity, proceeding analysis, letter opinions, a study without or inappropriate outcome variables, and a study reported in languages other than English or Korean.
Information sources and search strategy
The study performed a literature search for articles published until May 2020 in databases such as PubMed, Embase, and CINAHL. The study checked for the literature search. ("middle-aged" OR "older adult" OR "elderly" OR "aging") AND ("exercise" OR "training" OR "physical activity") AND ("sarcopenic obesity" OR ("sarcopenia" AND "obesity")) AND ("blood pressure" OR "glucose" OR "triglyceride" OR "cholesterol" OR "cardiometabolic" OR "cardiovascular" OR "weight" OR "waist circumference" OR "BMI" OR "muscle mass")
Study selection and data extraction
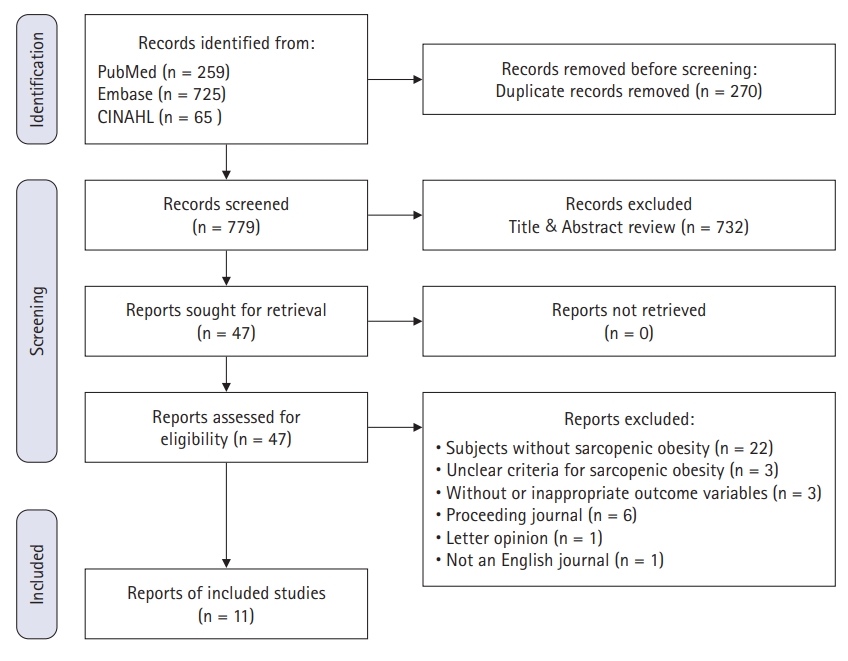
In May 2020, The study conducted the first literature search using search terms and strategies. One thousand forty-nine documents were searched, including PubMed 259, Embase 725, and CINAHL 65. Figure 1 presents the whole screening process of the study. There were 11 articles included in the study (Appendix 1).
Quality assessment
This study adopted the Cochrane Collaboration Risk of Bias tool for quality assessment [20]. The risk of bias assessment process was also enhanced by soliciting consultation from a fellow researcher, ensuring a more comprehensive and rigorous evaluation. Two investigators evaluated independently using the Cochrane Collaboration Risk of Bias tool and confirmed together. Assessment criteria were random sequence generation, allocation concealment, blinding participants and personnel, blinding outcome assessment, incomplete outcome data, and selective reporting.
Table 1 presents the assessment results. This study analyzed all included articles irrespective of the risk of bias due to the relevant article shortage.
RESULTS
Characteristics of participants and sarcopenic obesity
Out of eleven studies, eight were female participants, two were male participants, and one was male and female. The average age of participants was between 55 and 90 years old.
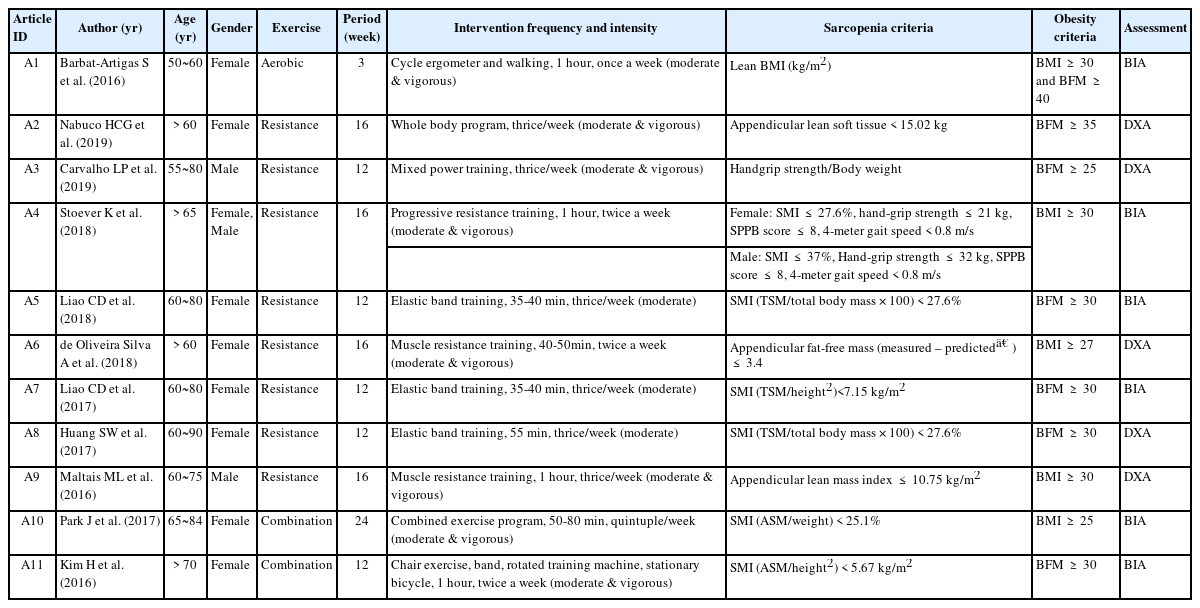
The studies use the indicators of sarcopenia and obesity to define sarcopenic obesity (Table 2). Various indicators were applied alone or in combination to define sarcopenia. Even though its calculation methods and diagnostic criteria differed, the skeletal muscle index (SMI) was the most prevalent sarcopenic indicator (6 studies). Obesity indicators were body mass index (BMI), body fat mass, and visceral fat area alone or in combination. Body fat mass (7 studies) was the most prevalent indicator, followed by BMI (5 studies). The body composition was measured by bioelectrical impedance analysis (6 studies) or dual-energy X-ray absorptiometry (5 studies).
Outcome variables
Outcome variables for intervention were muscle indicators, obesity indicators, physical function indicators, and cardiometabolic indicators (Table 3).
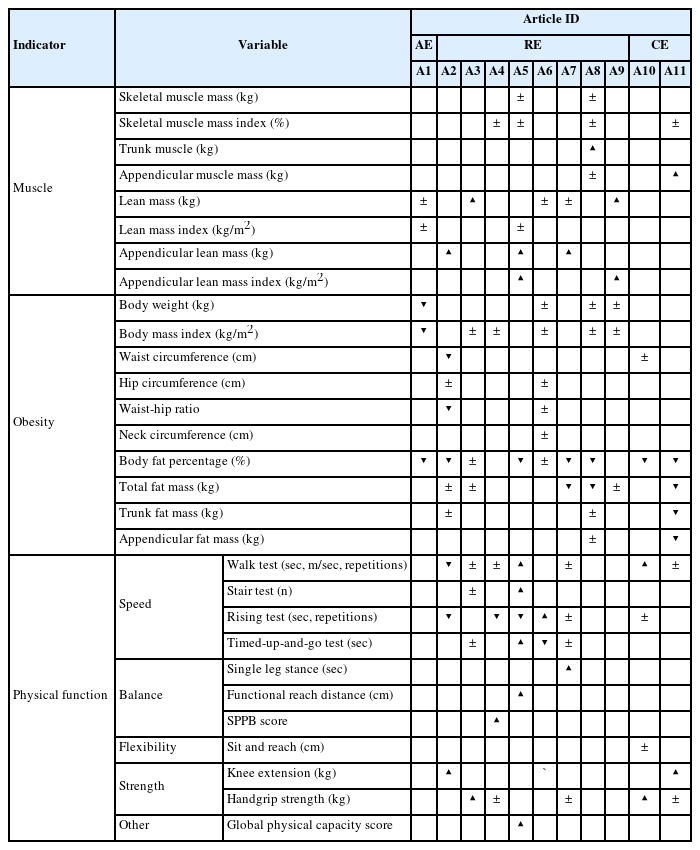
Lean mass (kg) and SMI (%) were prevalent as muscle indicators. Twenty-one muscle indicators were evaluated in ten studies (90.9%), of which nine (42.9%) showed significant changes. Most studies adopted BMI (kg/m2) or Body fat of total body or appendicular in percentage (%) or kilogram (kg) as obesity indicators. In the obesity indicator, there were significant changes in 16 out of 37 (43.2%) variables in eleven (100%) studies. Physical functions were categorized as speed, balance, flexibility, strength, etc. A walk test was the most prevalent for speed evaluation, followed by a rising test. Eight studies (72.7%) evaluated thirty-one physical function indicators, and eighteen (58.1%) showed significant results.
Six (54.5%) of the eleven studies evaluated cardiometabolic indicators such as blood pressure, serum glucose, cholesterol, an inflammatory cytokine, artery thickness, and blood flow velocity (Table 4). Of the forty-eight cardiometabolic indicators evaluated, sixteen showed significant results.
The Exercises and effects
Aerobic exercise
There was only one study that applied aerobic exercise. The study applied moderate- and vigorous-intensity exercise once a week for three weeks, and the session lasted one hour (Table 2). Among the obesity indicators, body weight, BMI, and body fat decreased. However, no significant changes in the muscle indicators and physical function were not evaluated (Table 3). Cardiometabolic indicators had improved in all measured variables, such as total cholesterol, triglyceride, low-density lipoprotein cholesterol, and systolic blood pressure, except for high-density lipoprotein cholesterol (Table 4).
Resistance exercise
Five out of eight studies applied moderate- and vigorous-intensity exercises. The other three studies applied moderate-intensity exercises. The minimum 35-minute to maximum one-hour exercise was performed twice or thrice a week for 12 to 16 weeks (Table 2).
In muscle indicators, appendicular lean mass or appendicular lean mass index increased in four out of eight resistance exercise studies. Still, there were no significant changes in all three studies evaluating skeletal muscle or SMI (Table 3). For the obesity indicator, six out of eight studies investigated a body fat percentage and reported a decrease in four of them. One study reported decreased waist circumference or waist-to-hip ratio. On the other hand, three and five studies measured body weight and BMI, respectively, but showed no significant changes (Table 3).
Six out of eight studies evaluated physical function indicators. Speed improved in four out of six studies. The study that evaluated the balance was three out of eight studies, and all three studies reported improvement. The strength was evaluated in four out of eight studies. One out of four studies reported enhanced handgrip strength. On the other hand, one study reported increased knee extension (Table 3).
Three out of eight studies evaluated cardiometabolic indicators. However, only one out of three studies reported improved cardiometabolic risks such as glucose, triglyceride, high-density lipoprotein cholesterol, High-sensitivity c-reactive protein, and Interleukin-6 (Table 4).
Combination exercise
Two studies adopted moderate- and vigorous-intensity combination exercise (Table 2). One study applied the 50- to 80-minute exercise five days per week for 24 weeks, and the other applied one-hour exercise twice weekly for twelve weeks.
The appendicular muscle mass as muscle indicators increased in one study. However, there were no significant changes in SMI (Table 3). For the obesity indicator, two studies all reported decreased body fat percentage. In addition, one out of two studies reported a decrease in total fat mass, trunk fat mass, and appendicular fat mass. On the other hand, only one study evaluated waist circumference, and no significant changes (Table 3).
Two studies evaluated physical function indicators. However, speed showed improvement in one study evaluated through the walk test. Enhanced strength was reported in both studies through the knee extension test and handgrip strength test, respectively (Table 3).
Only one out of two studies that evaluated cardiometabolic indicators reported improved systolic blood pressure, total cholesterol, carotid artery intima-media thickness, peak systolic flow velocity, end-diastolic flow velocity, and wall shear rate (Table 4).
DISCUSSION
This study investigated the definitions, diagnostic criteria, and measurement of sarcopenic obesity and the effective exercise interventions improving cardiometabolic outcomes of sarcopenic obesity for adults over middle-aged. Unfortunately, there were few attempts to manage sarcopenic obesity by relating it to cardiometabolic risk, and only eleven studies met the criteria. Identifying eleven articles in this study, concepts of sarcopenic obesity were different. Even though the same indicators were used to define sarcopenic obesity, it was difficult to compare the study results because the measurement methods and units were not the same. In addition, the cutoff values were all different.
Sarcopenic obesity, in which muscle loss and fat increase simultaneously, has various pathophysiological changes. Decreased skeletal muscle mass increases fragility and loss of independence associated with decreased muscle strength, impairing its role as an endocrine organ. As an endocrine organ, skeletal muscle release various myokines such as adiponectin, apelin, β‐aminoisobutyric acid, fibroblast growth factor 21, Interleukin-6, Interleukin‐10, Interleukin-15, irisin, myonectin, and musclin [9]. These myokines are involved in the metabolism of glucose, fat, inflammation, and cardiometabolic risk. Moreover, excessive proinflammatory cytokines from exacerbated adipose tissue alter adipokine expressions, which creates a high flow of lipids to muscle fibers [9,21]. The atrophy of skeletal muscle cells decreases the expression of glucose transporter type 4 in muscle fibers and reduces the demand for insulin-dependent glucose uptake [22]. The proinflammatory state and lipid accumulation in muscle fibers, on the other hand, induce the activity of intracellular kinases that phosphorylate and inactivate the insulin receptor and substrates [9]. Insulin resistance is a central mechanism for associating sarcopenic obesity with cardiovascular disease [21]. As such, sarcopenic obesity acts as a risk factor for cardiovascular disease. Therefore, to define sarcopenic obesity, it is necessary to consider cardiometabolic risk as well as muscle and obesity indicators. However, as can be seen from the results of this review, only six out of eleven studies (approximately 54.5%) adopted cardiometabolic indicators. In the case of studies employing resistance exercise, only one study considered cardiometabolic indicators.
Moreover, the application of exercise intervention for sarcopenic obesity should evaluate the increase in skeletal muscle and decrease in body fat based on the definition of sarcopenic obesity. However, while the decrease in body fat was assessed in ten studies, only four out of eleven studies evaluated the skeletal muscle mass or skeletal muscle mass index, and none showed significant results in increasing skeletal muscle. These results raise doubts about the suitability of intervention for sarcopenic obesity. Therefore, awareness of this still needs to be improved, and more research is necessary to establish diagnostic criteria for cardiometabolic risk for sarcopenic obesity.
So far, bioelectrical impedance analysis and dual-energy X-ray absorptiometry have been the most prevalent methods to measure muscle mass or body fat. However, it is also necessary to consider the ease of screening, such as tape measure. Calf or thigh circumference might be an alternative to muscle mass measurement [23-26]. Moreover, waist circumference is a good indicator of central obesity in the ratio of the hip circumference or height [27]. In particular, the waist to hip ratio or the waist to height ratio are indicators that can quickly identify the cardiometabolic risk caused by the problem of body fat distribution, which is easy to overlook in normal BMI [27-29]. Calf circumference is associated with cardiovascular risk factors, particularly insulin resistance and carotid atherosclerosis [30]. Calf circumference cutoff values for sarcopenia vary according to age, gender, and race. For Koreans over 70, the calf circumference cutoff was < 35 cm for men and < 33 cm for women [23]. For Japanese over 40 years old and Brazil over 60, the calf circumference cutoff was < 34 cm for men and < 33 cm [24,25]. In Indonesia, over 60 years of age, the calf circumference cutoff was male < 34 cm and female < 29 cm [26]. Also, a smaller thigh circumference is associated with increased cardiovascular and coronary heart disease and hypertension [31,32]. In American subjects 18 years of age or older, an increase in thigh circumference by 1 cm resulted in a 6% decrease in cardiovascular mortality [33]. Thus, these anthropometric indices enable early detection of sarcopenic obesity, including cardiometabolic risk. If thigh, calf circumference, and waist circumference in ratio to hip or height are managed together in daily life, it would be possible to prevent cardiovascular disease, significantly reducing medical expenses.
Even though there were exercise interventions for sarcopenic obesity, such as aerobic, resistance, and combination exercise, there were too few studies to compare the impact of the outcome variable in sarcopenic obesity subjects. However, Aerobic exercise effectively manages cardiometabolic risk by improving glycemic control, cardiorespiratory fitness, myocardial function, vascular endothelial function, visceral adiposity, inflammation, and dyslipidemia [34]. On the other hand, resistance exercise increases muscle mass by stimulating the entire muscle and muscle fiber hypertrophy [35]. In a weight loss program for older adults, combined aerobic and resistance exercise improved the participants' functional status while preserving lean mass [36]. Combination exercise benefits weight loss, fat loss, cardiovascular health, leptin levels, and aerobic capacity [37]. Thus, combination exercise may help improve body composition and cardiometabolic risks in obese older people, ultimately improving cardiovascular function and health [38].
Exercise interventions for sarcopenic obese subjects should reduce body fat through aerobic exercise and increase muscle mass and strength through resistance exercise to create a synergistic effect, consequently reducing cardiometabolic risk. In particular, improving lower extremity muscle health may help prevent and ameliorate sarcopenic obesity while lowering the risk associated with metabolic syndrome and premature death and may reduce certain lipid levels, particularly those related to the development of cardiovascular disease [22,39]. However, additional research should investigate which aerobic and resistance exercise ratio in combination exercise is an effective intervention for sarcopenic obesity
Also, considering exercise intensity and frequency would be necessary. Considering the age at which sarcopenic obesity is prevalent, it is necessary to consider practical exercise applications that can be adopted continuously without being excessive. In this review, There were significant changes in muscle, obesity, and physical function indicators in studies that applied moderate-intensity exercise. However, no significant changes were observed in cardiometabolic indicators, and even more were not evaluated. Additional research is needed.
This study has some limitations. This study tried to find out exercise intervention effects on sarcopenic obesity. However, there were few preliminary studies. Because of the relevant article shortage, this study included articles irrespective of the risk of bias. Moreover, some study results are not limited to exercise, including diet interventions. In addition, the limitation of restricting the search to English-language databases resulted in a narrow scope by excluding publications in languages other than English.
For this reason, it was difficult to verify the effect of pure exercise intervention. Therefore, it is necessary to be careful in interpreting the results. Nevertheless, this study may raise awareness of sarcopenic obesity and its management, seeking to clarify definitions and assessing methods while identifying exercise interventions to improve the cardiometabolic risks. Further research on management and its effect on sarcopenic obesity is needed.
CONCLUSION
This study addresses a pressing public health concern in the context of an aging population, acknowledges the unique challenges of sarcopenic obesity, and tries to clarify definitions and assessment methods while identifying effective exercise interventions to improve the cardiometabolic risks. Sarcopenic obesity is a multifaceted condition with varying definitions and diagnostic criteria. Its association with cardiometabolic risk underscores the need for comprehensive assessments considering both muscle and obesity indicators. While exercise interventions hold promise for managing sarcopenic obesity, further research is required to establish effective strategies.
Notes
CONFLICT OF INTEREST
The author declared that no conflict of interest.
AUTHORSHIP
HRK contributed to the conception and design of this study; HRK collected data; HRK performed the statistical analysis and interpretation; HRK drafted the manuscript; HRK critically revised the manuscript; HRK supervised the whole study process. The authors read and approved the final manuscript.
FUNDING
None.
DATA AVAILABILITY
The data supporting the findings of this study are available in the article and appendix.
References
Appendices
Appendix. List of reviewed articles for the study
A1. Barbat-Artigas S, Garnier S, Joffroy S, Riesco É, Sanguignol F, Vellas B, et al. Caloric restriction and aerobic exercise in sarcopenic and non-sarcopenic obese women: an observational and retrospective study. Journal of Cachexia, Sarcopenia and Muscle. 2016;7(3):284-289. http://doi.org/10.1002/jcsm.12075
A2. Nabuco HCG, Tomeleri CM, Fernandes RR, Sugihara Junior P, Cavalcante EF, Cunha PM, et al. Effect of whey protein supplementation combined with resistance training on body composition, muscular strength, functional capacity, and plasma-metabolism biomarkers in older women with sarcopenic obesity: a randomized, double-blind, placebo-controlled trial. Clinical Nutrition ESPEN. 2019;32:88-95. http://doi.org/10.1016/j.clnesp.2019.04.007
A3. Carvalho LP, Pion CH, Boutros GEH, Gaudreau P, Chevalier S, Bélanger M, et al. Effect of a 12-week mixed power training on physical function in dynapenic-obese older men: does severity of dynapenia matter? Aging Clinical and Experimental Research. 2019;31(7):977-984. http://doi.org/10.1007/s40520-018-1048-0
A4. Stoever K, Heber A, Eichberg S, Brixius K. Influences of resistance training on physical function in older, obese men and women with sarcopenia. Journal of Geriatric Physical Therapy. 2018;41(1):20-27. http://doi.org/10.1519/jpt.0000000000000105
A5. Liao CD, Tsauo JY, Huang SW, Ku JW, Hsiao DJ, Liou TH. Effects of elastic band exercise on lean mass and physical capacity in older women with sarcopenic obesity: a randomized controlled trial. Scientific Reports. 2018;8(1):2317. http://doi.org/10.1038/s41598-018-20677-7
A6. de Oliveira Silva A, Dutra MT, de Moraes W, Funghetto SS, Lopes de Farias D, Dos Santos PHF, et al. Resistance training-induced gains in muscle strength, body composition, and functional capacity are attenuated in elderly women with sarcopenic obesity. Clinical Intervention in Aging. 2018;13:411-417. http://doi.org/10.2147/cia.S156174
A7. Liao CD, Tsauo JY, Lin LF, Huang SW, Ku JW, Chou LC, et al. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity: a CONSORT-compliant prospective randomized controlled trial. Medicine. 2017;96(23):e7115. http://doi.org/10.1097/md.0000000000007115
A8. Huang SW, Ku JW, Lin LF, Liao CD, Chou LC, Liou TH. Body composition influenced by progressive elastic band resistance exercise of sarcopenic obesity elderly women: a pilot randomized controlled trial. European Journal of Physical and Rehabilitation Medicine. 2017;53(4):556-563. http://doi.org/10.23736/s1973-9087.17.04443-4
A9. Maltais ML, Perreault K, Courchesne-Loyer A, Lagacé JC, Barsalani R, Dionne IJ. Effect of resistance training and various sources of protein supplementation on body fat mass and metabolic profile in sarcopenic overweight older adult men: a pilot study. International Journal of Sport Nutrition and Exercise Metabolism. 2016;26(1):71-77. http://doi.org/10.1123/ijsnem.2015-0160
A10. Park J, Kwon Y, Park H. Effects of 24-week aerobic and resistance training on carotid artery intima-media thickness and flow velocity in elderly women with sarcopenic obesity. Journal of Atherosclerosis Thrombosis. 2017;24(11):1117-1124. http://doi.org/10.5551/jat.39065
A11. Kim H, Kim M, Kojima N, Fujino K, Hosoi E, Kobayashi H, et al. Exercise and nutritional supplementation on community-dwelling elderly Japanese women with sarcopenic obesity: a randomized controlled trial. Journal of the American Medical Directors Association. 2016;17(11):1011-1019. http://doi.org/10.1016/j.jamda.2016.06.016